Biomarkers Market By Type (safety,validation, Efficacy), Application (diagnostic, Drug Development, Personalized Medicine And Other Applications) And Disease (oncology Diseases, Immunological Disease, Neurological Disease, Cardiovascular Disease And Other Diseases)- Global Industry Analysis And Forecast To 2023
Published On : July 2018 Pages : 190 Category: Biotechnology Report Code : HC071088
Industry Outlook and Trend Analysis
The Biomarkers Market was worth USD 21.67 billion in 2017 and is expected to reach approximately USD 68.93 billion by 2023, while registering itself at a compound annual growth rate (CAGR) of 13.72% during the forecast period. Biomarkers are cellular or molecular diagnostic tools, estimated in biological samples, for example, urine, blood and saliva. It is used as a marker to measure and assess biological procedures, pathogenic processes and pharmacological response. Some of the main types of biomarkers are predictive, prognostic, pharmacodynamics and efficacy reaction biomarker. A wide range of biomarkers are accessible for biological systems, for example, immune system, metabolic system and cardiovascular system. Biomarkers are useful for the detection, monitor disease progression and predict disease susceptibility, metabolic diseases, cancer, central nervous system issues and immune system ailments.
Drivers and Restraints
As of late there is expanded usage of biomarkers because of increment in pervasiveness of different illnesses. The support from the FDA for biomarkers development and expanding interest of customized medication are a portion of the elements driving the development for worldwide biomarkers market. Additionally, usage of biomarkers by major pharmaceuticals companies to conquer expanding rates of failures for drugs in clinical trial phase II and III, and expanding drug advancement cost are additionally energizing the development for worldwide biomarkers market.
Market Segmentation
The Biomarkers Market is segmented on the basis of type, application and disease. Based on type the market is segmented into Safety, Validation and Efficacy. On the basis of application the market is segmented into Diagnostic, Personalized Medicine, Drug Development and Other Applications. On the basis of diseases the market is segmented into Oncology Diseases, Neurological Disease, Cardiovascular Disease, Immunological Disease and Other Diseases. Nonetheless, high capital investment needed for the application and high cost of validation, development and discovery of biomarkers is a portion of the components limiting the development for worldwide biomarkers market.
Regional Outlook and Trend Analysis
North America rules the worldwide market of biomarkers because of expanding interest of aged populace for analysis and treatment of age related illnesses. Moreover, higher rate of adoption of biomarkers technologies is likewise driving the development of biomarkers market in North America. Asia is anticipated to indicate high development rates in the following five years in worldwide biomarkers market. A portion of the elements driving the development of biomarkers market in developing markets of Asia are expanding innovative work movement in India and China, expanding number of agreement inquire about associations and low cost for carrying out clinical trials.
Competitive Insights
The leading players in the market are Roche Diagnostics, Qiagen, Merck & Co, Agilent Technologies, Siemens Healthineers, Abbott Laboratories, GE Healthcare and Johnson & Johnson Private Limited. The major players in the market are profiled in detail in view of qualities, for example, company portfolio, business strategies, financial overview, recent developments, and share of the overall industry.
The Biomakers Market is segmented on the basis of regions as follows-
By Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Russia
- Italy
- Rest of Europe
- Asia-Pacific
- China
- Japan
- South Korea
- India
- Southeast Asia
- Rest of Asia-Pacific
- South America
- Brazil
- Argentina
- Columbia
- South Africa
- Rest of South America
- Middle East and Africa
- Saudi Arabia
- UAE
- Egypt
- Nigeria
- South Africa
- Rest of MEA
Some of the key questions answered by the report are:
- What was the market size in 2017 and forecast from 2017 to 2023?
- What will be the industry market growth from 2017 to 2023?
- What are the major drivers, restraints, opportunities, challenges, and industry trends and their impact on the market forecast?
- What are the major segments leading the market growth and why?
- Which are the leading players in the market and what are the major strategies adopted by them to sustain the market competition?
Market Classification
· Biomarkers Market, By Application, Estimates and Forecast, 2014-2023($Billion)
o Diagnostic
o Drug Development
o Personalized Medicine
o Other Applications
· Biomarkers Market, By Type, Estimates and Forecast, 2014-2023($Billion)
o Safety
o Validation
o Efficacy
· Biomarkers Market, By Disease, Estimates and Forecast, 2014-2023($Billion)
o Oncology Diseases
o Immunological Disease
o Neurological Disease
o Cardiovascular Disease
o Other Diseases
· Biomarkers Market, By Region, Estimates and Forecast, 2014-2023($Billion)
o North America
§ North America Biomarkers Market, By Country
o U.S. Biomarkers Market
o Canada Biomarkers Market
o Mexico Biomarkers Market
o Europe
§ Europe Biomarkers Market, By Country
o Germany Biomarkers Market
o UK Biomarkers Market
o France Biomarkers Market
o Russia Biomarkers Market
o Italy Biomarkers Market
o Rest of Europe Biomarkers Market
o Asia-Pacific
§ Asia-Pacific Biomarkers Market, By Country
o China Biomarkers Market
o Japan Biomarkers Market
o South Korea Biomarkers Market
o India Biomarkers Market
o Southeast Asia Biomarkers Market
o Rest of Asia-Pacific Biomarkers Market
o South America
§ South America Biomarkers Market
o Brazil Biomarkers Market
o Argentina Biomarkers Market
o Columbia Biomarkers Market
o South Africa Biomarkers Market
o Rest of South America Biomarkers Market
o Middle East and Africa
§ Middle East and Africa Biomarkers Market
o Saudi Arabia Biomarkers Market
o UAE Biomarkers Market
o Egypt Biomarkers Market
o Nigeria Biomarkers Market
o South Africa Biomarkers Market
o Rest of MEA Biomarkers Market
Table of Contents
1. Introduction
1.1. Report Description
1.2. Research Methodology
1.2.1. Secondary Research
1.2.2. Primary Research
2. Executive Summary
2.1. Key Highlights
3. Market Overview
3.1. Introduction
3.1.1. Market Definition
3.1.2. Market Segmentation
3.2. Market Dynamics
3.2.1. Drivers
3.2.2. Restraints
3.2.3. Opportunities
4. Market Analysis by Regions
4.1. North America (United States, Canada and Mexico)
4.1.1. United States Market States and Outlook (2017-2023)
4.1.2. Canada Market States and Outlook (2017-2023)
4.1.3. Mexico Market States and Outlook (2017-2023)
4.2. Europe (Germany, France, UK, Russia, Italy and Rest of Europe)
4.2.1. Germany Market States and Outlook (2017-2023)
4.2.2. France Market States and Outlook (2017-2023)
4.2.3. UK Market States and Outlook (2017-2023)
4.2.4. Russia Market States and Outlook (2017-2023)
4.2.5. Italy Market States and Outlook (2017-2023)
4.2.6. Rest of Europe Market States and Outlook (2017-2023)
4.3. Asia-Pacific (China, Japan, Korea, India, Southeast Asia and Rest of Asia-Pacific)
4.3.1. China Market States and Outlook (2017-2023)
4.3.2. Japan Market States and Outlook (2017-2023)
4.3.3. Korea Market States and Outlook (2017-2023)
4.3.4. India Market States and Outlook (2017-2023)
4.3.5. Rest of Asia-Pacific Market States and Outlook (2017-2023)
4.4. South America (Brazil, Argentina, Columbia and Rest of South America)
4.4.1. Brazil Market States and Outlook (2017-2023)
4.4.2. Argentina Market States and Outlook (2017-2023)
4.4.3. Columbia Market States and Outlook (2017-2023)
4.4.4. Rest of South America Market States and Outlook (2017-2023)
4.5. Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria, South Africa and Rest of MEA)
4.5.1. Saudi Arabia Market States and Outlook (2017-2023)
4.5.2. UAE Market States and Outlook (2017-2023)
4.5.3. Egypt Market States and Outlook (2017-2023)
4.5.4. Nigeria Market States and Outlook (2017-2023)
4.5.5. South Africa Market States and Outlook (2017-2023)
4.5.6. Rest of MEA Market States and Outlook (2017-2023)
5. Biomarkers Market, By Type
5.1. Introduction
5.2. Global Biomarkers Revenue and Market Share by Type (2017-2027)
5.2.1. Global Biomarkers Revenue and Revenue Share by Type (2017-2027)
5.3. Safety
5.3.1. Global Safety Revenue and Growth Rate (2017-2027)
5.4. Validation
5.4.1. Global Validation Revenue and Growth Rate (2017-2027)
5.5. Efficacy
5.5.1. Global Efficacy Revenue and Growth Rate (2017-2027)
6. Biomarkers Market, By Application
6.1. Introduction
6.2. Global Biomarkers Revenue and Market Share by Application (2017-2027)
6.2.1. Global Biomarkers Revenue and Revenue Share by Application (2017-2027)
6.3. Diagnostic
6.3.1. Global Diagnostic Revenue and Growth Rate (2017-2027)
6.4. Drug Development
6.4.1. Global Drug Development Revenue and Growth Rate (2017-2027)
6.5. Personalized Medicine
6.5.1. Global Personalized Medicine Revenue and Growth Rate (2017-2027)
6.6. Other Applications
6.6.1. Global Other Applications Revenue and Growth Rate (2017-2027)
7. Biomarkers Market, By Disease
7.1. Introduction
7.2. Global Biomarkers Revenue and Market Share by Disease (2017-2027)
7.2.1. Global Biomarkers Revenue and Revenue Share by Disease (2017-2027)
7.3. Oncology Diseases
7.3.1. Global Oncology Diseases Revenue and Growth Rate (2017-2027)
7.4. Immunological Disease
7.4.1. Global Immunological Disease Revenue and Growth Rate (2017-2027)
7.5. Neurological Disease
7.5.1. Global Neurological Disease Revenue and Growth Rate (2017-2027)
7.6. Cardiovascular Disease
7.6.1. Global Cardiovascular Disease Revenue and Growth Rate (2017-2027)
7.7. Other Diseases
7.7.1. Global Other Diseases Revenue and Growth Rate (2017-2027)
8. Biomarkers Market, By Region
8.1. Introduction
8.2. Global Biomarkers Revenue and Market Share by Regions
8.2.1. Global Biomarkers Revenue by Regions (2017-2027)
8.3. North America Biomarkers by Countries
8.3.1. North America Biomarkers Revenue and Growth Rate (2017-2027)
8.3.2. North America Biomarkers Revenue by Countries (2017-2027)
8.3.3. North America Biomarkers Revenue (Million USD) by Countries (2017-2027)
8.3.4. U.S.
8.3.4.1. United States Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.3.5. Canada
8.3.5.1. Canada Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.3.6. Mexico
8.3.6.1. Mexico Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.4. Europe Biomarkers by Countries
8.4.1. Europe Biomarkers Revenue and Growth Rate (2017-2027)
8.4.2. Europe Biomarkers Revenue by Countries (2017-2027)
8.4.3. Europe Biomarkers Revenue (Million USD) by Countries (2017-2027)
8.4.4. Germany
8.4.4.1. Germany Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.4.5. UK
8.4.5.1. UK Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.4.6. France
8.4.6.1. France Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.4.7. Russia
8.4.7.1. Russia Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.4.8. Italy
8.4.8.1. Italy Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.4.9. Rest of Europe
8.4.9.1. Rest of Europe Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.5. Asia-Pacific
8.5.1. Asia-Pacific Biomarkers Revenue and Growth Rate (2017-2027)
8.5.2. Asia-Pacific Biomarkers Revenue by Countries (2017-2027)
8.5.3. Asia-Pacific Biomarkers Revenue (Million USD) by Countries (2017-2027)
8.5.4. China
8.5.4.1. China Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.5.5. Japan
8.5.5.1. Japan Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.5.6. Korea
8.5.6.1. Korea Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.5.7. India
8.5.7.1. India Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.5.8. Southeast Asia
8.5.8.1. Southeast Asia Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.5.9. Rest of Asia-Pacific
8.5.9.1. Rest of Asia-Pacific Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.6. South America
8.6.1. South America Biomarkers Revenue and Growth Rate (2017-2027)
8.6.2. South America Biomarkers Revenue by Countries (2017-2027)
8.6.3. South America Biomarkers Revenue (Million USD) by Countries (2017-2027)
8.6.4. Brazil
8.6.4.1. Brazil Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.6.5. Argentina
8.6.5.1. Argentina Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.6.6. Columbia
8.6.6.1. Columbia Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.6.7. Rest of South America
8.6.7.1. Rest of South America Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.7. Middle East and Africa
8.7.1. Middle East and Africa Biomarkers Revenue and Growth Rate (2017-2027)
8.7.2. Middle East and Africa Biomarkers Revenue by Countries (2017-2027)
8.7.3. Middle East and Africa Biomarkers Revenue (Million USD) by Countries (2017-2027)
8.7.4. Saudi Arabia
8.7.4.1. Saudi Arabia Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.7.5. United Arab Emirates
8.7.5.1. United Arab Emirates Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.7.6. Egypt
8.7.6.1. Egypt Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.7.7. Nigeria
8.7.7.1. Nigeria Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.7.8. South Africa
8.7.8.1. South Africa Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
8.7.9. Rest of Middle East and Africa
8.7.9.1. Rest of Middle East and Africa Biomarkers Revenue (Millions USD) and Growth Rate (2017-2027)
9. Company Profiles
9.1. Roche Diagnostics Ltd.
9.1.1. Business Overview
9.1.2. Type Portfolio
9.1.3. Strategic Developments
9.1.4. Revenue and Market Share
9.2. Agilent Technologies, Inc.
9.2.1. Business Overview
9.2.2. Type Portfolio
9.2.3. Strategic Developments
9.2.4. Revenue and Market Share
9.3. Abbott
9.3.1. Business Overview
9.3.2. Type Portfolio
9.3.3. Strategic Developments
9.3.4. Revenue and Market Share
9.4. Johnson & Johnson Private Limited
9.4.1. Business Overview
9.4.2. Type Portfolio
9.4.3. Strategic Developments
9.4.4. Revenue and Market Share
9.5. GE Healthcare
9.5.1. Business Overview
9.5.2. Type Portfolio
9.5.3. Strategic Developments
9.5.4. Revenue and Market Share
9.6. Siemens Healthcare GmbH
9.6.1. Business Overview
9.6.2. Type Portfolio
9.6.3. Strategic Developments
9.6.4. Revenue and Market Share
9.7. Merck & Co., Inc.
9.7.1. Business Overview
9.7.2. Type Portfolio
9.7.3. Strategic Developments
9.7.4. Revenue and Market Share
9.8. Qiagen
9.8.1. Business Overview
9.8.2. Type Portfolio
9.8.3. Strategic Developments
9.8.4. Revenue and Market Share
10. Global Biomarkers Market Competition, by Manufacturer
10.1. Global Biomarkers Revenue and Market Share by Manufacturer (2017-2017)
10.2. Global Biomarkers Price by Region (2017-2017)
10.3. Top 5 Biomarkers Manufacturer Market Share
10.4. Market Competition Trend
11. Biomarkers Market Forecast (2027-2023)
11.1. Global Biomarkers Revenue (Millions USD) and Growth Rate (2027-2023)
11.2. Biomarkers Market Forecast by Regions (2027-2023)
11.2.1. North America Biomarkers Market Forecast (2027-2023)
11.2.1.1. United States Biomarkers Market Forecast (2027-2023)
11.2.1.2. Canada Biomarkers Market Forecast (2027-2023)
11.2.1.3. Mexico Biomarkers Market Forecast (2027-2023)
11.2.2. Europe Biomarkers Market Forecast (2027-2023)
11.2.2.1. Germany Biomarkers Market Forecast (2027-2023)
11.2.2.2. United Kingdom Biomarkers Market Forecast (2027-2023)
11.2.2.3. France Biomarkers Market Forecast (2027-2023)
11.2.2.4. Russia Biomarkers Market Forecast (2027-2023)
11.2.2.5. Italy Biomarkers Market Forecast (2027-2023)
11.2.2.6. Rest of the Europe Biomarkers Market Forecast (2027-2023)
11.2.3. Asia-Pacific Biomarkers Market Forecast (2027-2023)
11.2.3.1. China Biomarkers Market Forecast (2027-2023)
11.2.3.2. Japan Biomarkers Market Forecast (2027-2023)
11.2.3.3. Korea Biomarkers Market Forecast (2027-2023)
11.2.3.4. India Biomarkers Market Forecast (2027-2023)
11.2.3.5. Southeast Asia Biomarkers Market Forecast (2027-2023)
11.2.3.6. Rest of Asia-Pacific Biomarkers Market Forecast (2027-2023)
11.2.4. South America Biomarkers Market Forecast (2027-2023)
11.2.4.1. Brazil Biomarkers Market Forecast (2027-2023)
11.2.4.2. Argentina Biomarkers Market Forecast (2027-2023)
11.2.4.3. Columbia Biomarkers Market Forecast (2027-2023)
11.2.4.4. Rest of South America Biomarkers Market Forecast (2027-2023)
11.2.5. Middle East and Africa Biomarkers Market Forecast (2027-2023)
11.2.5.1. Saudi Arabia Biomarkers Market Forecast (2027-2023)
11.2.5.2. UAE Biomarkers Market Forecast (2027-2023)
11.2.5.3. Egypt Biomarkers Market Forecast (2027-2023)
11.2.5.4. Nigeria Biomarkers Market Forecast (2027-2023)
11.2.5.5. South Africa Biomarkers Market Forecast (2027-2023)
11.2.5.6. Rest of MEA Biomarkers Market Forecast (2027-2023)
11.3. Biomarkers Market Forecast by Type (2027-2023)
11.3.1. Global Biomarkers Revenue Forecast by Type (2027-2023)
11.3.2. Global Biomarkers Revenue Market Share Forecast by Type (2027-2023)
11.4. Biomarkers Market Forecast by Application (2027-2023)
11.4.1. Global Biomarkers Revenue Forecast by Application (2027-2023)
11.4.2. Global Biomarkers Revenue Market Share Forecast by Application (2027-2023)
11.5. Biomarkers Market Forecast by Disease (2027-2023)
11.5.1. Global Biomarkers Revenue Forecast by Disease (2027-2023)
11.5.2. Global Biomarkers Revenue Market Share Forecast by Disease (2027-2023)
List of Tables
*You can glance through the list of Tables and Figures when you view the sample copy of Biomarkers Market.
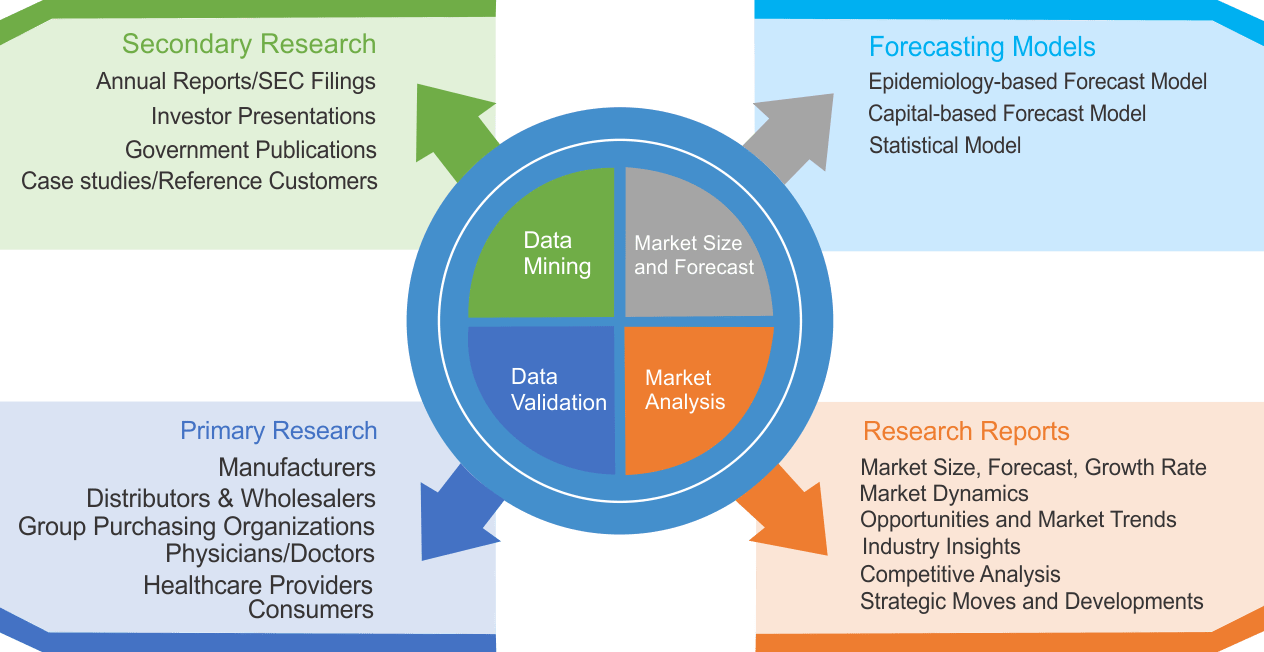
Research Methodology
We use both primary as well as secondary research for our market surveys, estimates and for developing forecast. Our research process commence by analyzing the problem which enable us to design the scope for our research study. Our research process is uniquely designed with enough flexibility to adjust according to changing nature of products and markets, while retaining core element to ensure reliability and accuracy in research findings. We understand both macro and micro-economic factors to evaluate and forecast different market segments.
Data Mining
Data is extensively collected through various secondary sources such as annual reports, investor presentations, SEC filings, and other corporate publications. We also refer trade magazines, technical journals, paid databases such as Factiva and Bloomberg, industry trade journals, scientific journals, and social media data to understand market dynamics and industry trends. Further, we also conduct primary research to understand market drivers, restraints, opportunities, challenges, and competitive scenario to build our analysis.
Data Collection Matrix
|
Data Collection Matrix |
Supply Side |
Demand Side |
|
Primary Data Sources |
|
|
|
Secondary Data Sources |
|
|
Market Modeling and Forecasting
We use epidemiology and capital equipment-based models to forecast market size of different segments at country and regional level.
- Epidemiology-based Forecasting Model: This method uses epidemiology data gathered through various publications and from physicians to estimate population of patients, flow of treatment of individual disease and therapies. The data collected through this method includes statics on incidence of disease, population suffering from disease, and treatment population. This method is used to understand:
- Number of patients for particular device or medical procedure and
- Repeated use of particular device depending on health and condition of patient
- Capital-based Forecasting Model: This method of forecasting is based on number of replacements, installed-based and new sales of capital equipment used in various healthcare and diagnostic centers. These three parameters are calculated and forecast is developed. Installation base is calculated as average number of units per facility; while sales for particular year is calculated from number of new and replace units. Secondary data is collected through various supply chain intermediaries and opinion leaders to arrive at installation and sales rate. These techniques help our analysts in validating market and developed market estimates and forecast.
We do forecast on basis of several parameters such as market drivers, market opportunities, industry trends government regulations, raw materials supply and trade dynamics to ensure relevance of forecast with market scenario. With increasing need to granulized information, we used bottom-up methodology for forecasting where we evaluate each regional segment differently and combined all forecast to develop final market forecast.
Data Validation
We believe primary research is a very important tool in analyzing and forecasting different markets. In order to make sure accuracy of our findings, our team conducts primary interviews at every stage of research to gain deep insights into current business environment and future trends and key developments in market. This includes use of various methods such as telephonic interviews, focus groups, face to face interviews and questionnaires to validate our research from all aspects. We validate our data through primary research from key industry leaders such as CEO, product managers, marketing managers, suppliers, distributors, and consumers are frequently interviewed. These interviews provide valuable insights which help us to have better market understanding besides validating our estimates and forecast.
Data Triangulation
Industry Analysis
|
Qualitative Data |
Quantitative Data (2017-2025) |
|
|