.png)
Intrauterine Devices Market by Product Type - Global Industry Analysis and Forecast to 2023
Published On : January 2018 Pages : 102 Category: Pharmaceuticals Report Code : HC01503
SEGMENTS & REGIONS:
- Regions: North America, Europe, Asia- Pacific, Latin America, Middle East & Africa
Industry Outlook and Trend Analysis
The global Intrauterine Devices Market was worth USD billion in 2017 and is expected to reach approximately USD billion by 2023, while registering itself at a compound annual growth rate (CAGR) of % during the forecast period. Intrauterine devices are contraceptive devices, frequently T-shaped, containing either levonorgestrel or copper, which are infused into the uterus. They are a successful type of reversible anti-conception medication and are a type of long-acting contraception. This market is anticipated to encounter significant development amid the gauge time frame and is impacted by the distinctive local patterns that fragment the market with regards to the accessibility of various contraceptive devices. Upcoming patterns, for example, the developing rate of unintended pregnancies are rapidly growing as it controls unwarranted healthcare expenditure on abortions.
Product type outlook and Trend Analysis
The Global Intrauterine Devices market is segmented on the basis of product type into Copper intrauterine device and Hormonal intrauterine device. Hormonal intrauterine devices are evaluated to have a significant share of the market. Since hormonal intrauterine devices have less difficulties and dangers contrasted with non-hormonal intrauterine devices.
Regional Outlook and Trend Analysis
Geographically, the EMEA area is evaluated to be the biggest market for IUDs amid the gauge time frame. This district presently ruling the worldwide market for IUDs and is evaluated to hold its ruling hold over the market amid the conjecture time frame because of developing support from the administration and the increased arrangement of key organizations together amongst local and global organizations.
Competitive Insights
The leading layers in the market are Bayer Cropscience Limited, Agile Therapeutics, Lupin Pharmaceuticals Inc, Pfizer, ANI Pharmaceuticals Inc, Teva Pharmaceuticals, Effik International, Besins Healthcare, Brecuro Medical OÜ, Eurogine, Actavis and HRA Pharma SA. The major players in the market are profiled in detail in view of qualities, for example, company portfolio, business strategies, financial overview, recent developments, and share of the overall industry.
The Intrauterine devices Market is segmented as follows-
By Product Type:
- Copper Intrauterine Devices
- Hormonal Intrauterine Devices
By Region
- North America
- U.S
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia-Pacific
- Japan
- China
- Australia
- India
- South Korea
- Rest of Asia Pacific
- Rest of the World
- Brazil
- South Africa
- Saudi Arabia
- United Arab Emirates
- Others
Some of the key questions answered by the report are:
- What was the market size in 2017 and forecast from 2017 to 2023?
- What will be the industry market growth from 2017 to 2023?
- What are the major drivers, restraints, opportunities, challenges, and industry trends and their impact on the market forecast?
- What are the major segments leading the market growth and why?
- Which are the leading players in the market and what are the major strategies adopted by them to sustain the market competition?
Market Classification
- Intrauterine Devices Market, By Product Type, Estimates and Forecast, 2014-2023 ($Million)
- Copper Intrauterine Devices
- Hormonal Intrauterine Devices
- Intrauterine Devices Market, By Region, Estimates and Forecast, 2014-2023 ($Million)
- North America
- North America Intrauterine Devices Market, By Country
- North America Intrauterine Devices Market, By Product Type
- U.S. Intrauterine Devices Market, By Product Type
- Canada Intrauterine Devices Market, By Product Type
- Mexico Intrauterine Devices Market, By Product Type
-
- Europe
- Europe Intrauterine Devices Market, By Country
- Europe Intrauterine Devices Market, By Product Type
- Germany Intrauterine Devices Market, By Product Type
- France Intrauterine Devices Market, By Product Type
- UK Intrauterine Devices Market, By Product Type
- Italy Intrauterine Devices Market, By Product Type
- Spain Intrauterine Devices Market, By Product Type
- Rest of Europe Intrauterine Devices Market, By Product Type
-
- Asia-Pacific
- Asia-Pacific Intrauterine Devices Market, By Country
- Asia-Pacific Intrauterine Devices Market, By Product Type
- Japan Intrauterine Devices Market, By Product Type
- Australia Intrauterine Devices Market, By Product Type
- India Intrauterine Devices Market, By Product Type
- South Korea Intrauterine Devices Market, By Product Type
- Rest of Asia-Pacific Intrauterine Devices Market, By Product Type
- Asia-Pacific
-
- Rest of the World
- Rest of the World Intrauterine Devices Market, By Country
- Rest of the World Intrauterine Devices Market, By Product Type
- Brazil Intrauterine Devices Market, By Product Type
- South Africa Intrauterine Devices Market, By Product Type
- Saudi Arabia Intrauterine Devices Market, By Product Type
- Turkey Intrauterine Devices Market, By Product Type
- United Arab Emirates Intrauterine Devices Market, By Product Type
- Others Intrauterine Devices Market, By Product Type
- Rest of the World
Table of Contents
1. Introduction
1.1. Report Description
1.2. Research Methodology
1.2.1. Secondary Research
1.2.2. Primary Research
2. Executive Summary
2.1. Key Highlights
3. Market Overview
3.1. Introduction
3.1.1. Market Definition
3.1.2. Market Segmentation
3.2. Market Dynamics
3.2.1. Drivers
3.2.2. Growing demand for fertilizers
3.2.3. Restraints
3.2.3.1. Stringent Government regulations
3.2.4. Opportunities
4. Intrauterine Devices Market , By Product Type
4.1. Introduction
4.2. Intrauterine Devices Market Assessment and Forecast, By Product Type, 2017-2023
4.2.1. Copper Intrauterine Devices
4.2.2. Market Assessment and Forecast, By Region, 2017-2023 ($Million)
4.3. Hormonal Intrauterine Devices
4.3.1. Market Assessment and Forecast, By Region, 2017-2023 ($Million)
5. Intrauterine Devices Market , By Region
5.1. Introduction
5.2. Intrauterine Devices Market Assessment and Forecast, By Region, 2017-2023 ($Million)
5.3. North America
5.3.1. Market Assessment and Forecast, By Country, 2017-2023 ($Million)
5.3.2. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.3.3. U.S.
5.3.3.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.3.4. Canada
5.3.4.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.3.5. Mexico
5.3.5.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.4. Europe
5.4.1. Market Assessment and Forecast, By Country, 2017-2023 ($Million)
5.4.2. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.4.3. Germany
5.4.3.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.4.4. France
5.4.4.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.4.5. UK
5.4.5.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.4.6. Italy
5.4.6.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.4.7. Spain
5.4.7.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.4.8. Rest of Europe
5.4.8.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.5. Asia-Pacific
5.5.1. Market Assessment and Forecast, By Country, 2017-2023 ($Million)
5.5.2. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.5.3. Japan
5.5.3.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.5.4. China
5.5.4.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.5.5. Australia
5.5.5.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.5.6. India
5.5.6.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.5.7. South Korea
5.5.7.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.5.8. Rest of Asia-Pacific
5.5.8.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.6. Rest of the World
5.6.1. Market Assessment and Forecast, By Country, 2017-2023 ($Million)
5.6.2. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.6.3. Brazil
5.6.3.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.6.4. Turkey
5.6.4.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.6.5. Saudi Arabia
5.6.5.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.6.6. South Africa
5.6.6.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.6.7. United Arab Emirates
5.6.7.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
5.6.8. Others
5.6.8.1. Market Assessment and Forecast, By Product Type, 2017-2023 ($Million)
6. Company Profiles
6.1. Bayer Cropscience LimitedBusiness Overview
6.1.1. Product Portfolio
6.1.2. Key Financials
6.1.3. Strategic Developments
6.2. Agile TherapeuticsBusiness Overview
6.2.1. Product Portfolio
6.2.2. Key Financials
6.2.3. Strategic Developments
6.3. Lupin Pharmaceuticals Inc
6.3.1. Business Overview
6.3.2. Product Portfolio
6.3.3. Key Financials
6.3.4. Strategic Developments
6.4. Pfizer
6.4.1. Business Overview
6.4.2. Product Portfolio
6.4.3. Strategic Developments
6.5. ANI Pharmaceuticals Inc
6.5.1. Business Overview
6.5.2. Product Portfolio
6.5.3. Strategic Developments
6.6. Teva Pharmaceutical
6.6.1. Business Overview
6.6.2. Product Portfolio
6.6.3. Strategic Developments
6.7. Effik International
6.7.1. Business Overview
6.7.2. Product Portfolio
6.7.3. Strategic Developments
6.8. Besins Healthcare
6.8.1. Business Overview
6.8.2. Product Portfolio
6.8.3. Strategic Developments
6.9. Brecuro Medical OÜ
6.9.1. Business Overview
6.9.2. Product Portfolio
6.9.3. Strategic Developments
6.10. Actavis
6.10.1. Business Overview
6.10.2. Product Portfolio
6.10.3. Strategic Developments
6.11. HRA Pharma SA
6.11.1. Business Overview
6.11.2. Product Portfolio
6.11.3. Strategic Developments
List of Tables
Table 1.Global Intrauterine Devices Market, By Product Type ($Million), 2017-2023
Table 2.Copper Intrauterine Devices Market, By Region ($Million), 2017-2023
Table 3.Hormonal Intrauterine Devices Market, By Region ($Million), 2017-2023
Table 4.Intrauterine Devices Market, By Region ($Million), 2017-2023
Table 5.North America Intrauterine Devices Market, By Country, 2017-2023 ($Million)
Table 6.North America Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 7.U.S. Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 8.Canada Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 9.Mexico Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 10.Europe Intrauterine Devices Market, By Country, 2017-2023 ($Million)
Table 11.Europe Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 12.Germany Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 13.France Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 14.UK Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 15.Italy Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 16.Spain Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 17.Rest of Europe Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 18.Asia-Pacific Intrauterine Devices Market, By Country, 2017-2023 ($Million)
Table 19.Asia-Pacific Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 20.Japan Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 21.China Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 22.Australia Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 23.India Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 24.South Korea Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 25.Rest of Asia-Pacific Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 26.Rest of the World Intrauterine Devices Market, By Country, 2017-2023 ($Million)
Table 27.Rest of the World Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 28.Brazil Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 29.Turkey Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 30.Saudi Arabia Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 31.South Africa Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 32.United Arab Emirates Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 33.Others Intrauterine Devices Market, By Product Type, 2017-2023 ($Million)
Table 34.Bayer Cropscience Limited.: Key Strategic Developments, 2017-2017
Table 35 Agile Therapeutics: Key Strategic Developments, 2017-2017
Table 36.Lupin Pharmaceuticals Inc: Key Strategic Developments, 2017-2017
Table 37.Pfizer: Key Strategic Developments, 2017-2017
Table 38.J. ANI Pharmaceuticals Inc: Key Strategic Developments, 2017-2017
Table 39. Teva Pharmaceutical: Key Strategic Developments, 2017-2017
Table 40.Effik International: Key Strategic Developments, 2017-2017
Table 41.Besins Healthcare: Key Strategic Developments, 2017-2017
Table 42.Brecuro Medical OÜ: Key Strategic Developments, 2017-2017
Table 43.Actavis: Key Strategic Developments, 2017-2017
Table 44.HRA Pharma SA: Key Strategic Developments, 2017-2017
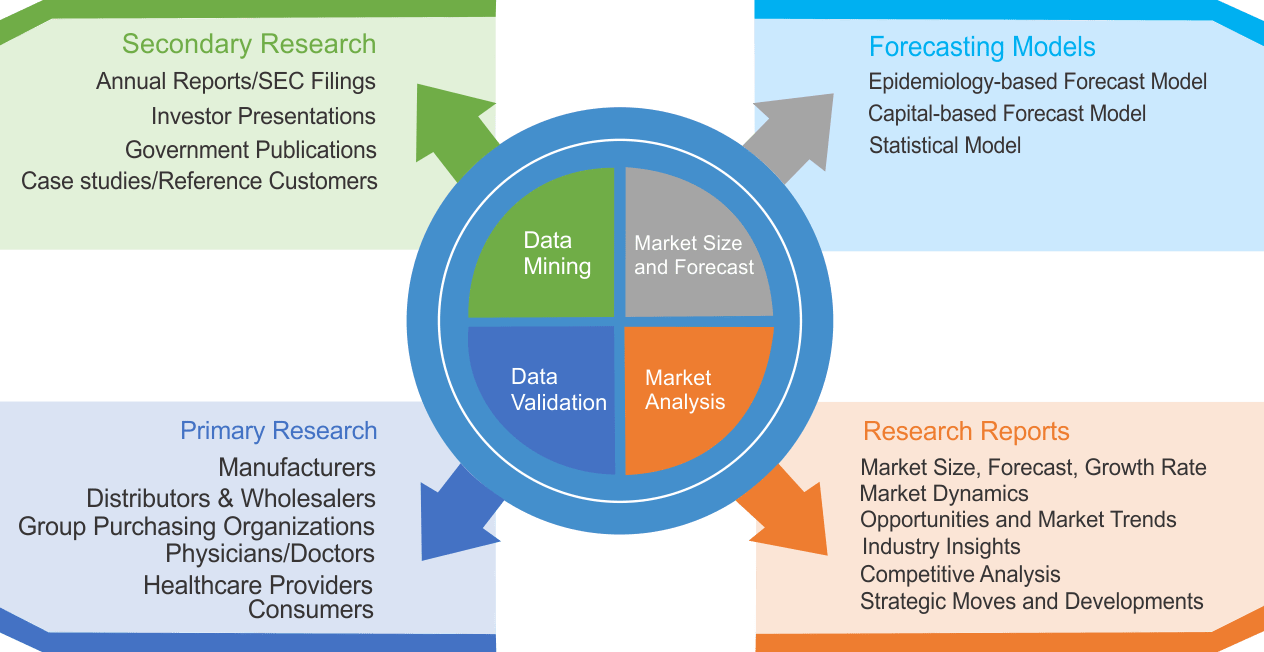
Research Methodology
We use both primary as well as secondary research for our market surveys, estimates and for developing forecast. Our research process commence by analyzing the problem which enable us to design the scope for our research study. Our research process is uniquely designed with enough flexibility to adjust according to changing nature of products and markets, while retaining core element to ensure reliability and accuracy in research findings. We understand both macro and micro-economic factors to evaluate and forecast different market segments.
Data Mining
Data is extensively collected through various secondary sources such as annual reports, investor presentations, SEC filings, and other corporate publications. We also refer trade magazines, technical journals, paid databases such as Factiva and Bloomberg, industry trade journals, scientific journals, and social media data to understand market dynamics and industry trends. Further, we also conduct primary research to understand market drivers, restraints, opportunities, challenges, and competitive scenario to build our analysis.
Data Collection Matrix
|
Data Collection Matrix |
Supply Side |
Demand Side |
|
Primary Data Sources |
|
|
|
Secondary Data Sources |
|
|
Market Modeling and Forecasting
We use epidemiology and capital equipment-based models to forecast market size of different segments at country and regional level.
- Epidemiology-based Forecasting Model: This method uses epidemiology data gathered through various publications and from physicians to estimate population of patients, flow of treatment of individual disease and therapies. The data collected through this method includes statics on incidence of disease, population suffering from disease, and treatment population. This method is used to understand:
- Number of patients for particular device or medical procedure and
- Repeated use of particular device depending on health and condition of patient
- Capital-based Forecasting Model: This method of forecasting is based on number of replacements, installed-based and new sales of capital equipment used in various healthcare and diagnostic centers. These three parameters are calculated and forecast is developed. Installation base is calculated as average number of units per facility; while sales for particular year is calculated from number of new and replace units. Secondary data is collected through various supply chain intermediaries and opinion leaders to arrive at installation and sales rate. These techniques help our analysts in validating market and developed market estimates and forecast.
We do forecast on basis of several parameters such as market drivers, market opportunities, industry trends government regulations, raw materials supply and trade dynamics to ensure relevance of forecast with market scenario. With increasing need to granulized information, we used bottom-up methodology for forecasting where we evaluate each regional segment differently and combined all forecast to develop final market forecast.
Data Validation
We believe primary research is a very important tool in analyzing and forecasting different markets. In order to make sure accuracy of our findings, our team conducts primary interviews at every stage of research to gain deep insights into current business environment and future trends and key developments in market. This includes use of various methods such as telephonic interviews, focus groups, face to face interviews and questionnaires to validate our research from all aspects. We validate our data through primary research from key industry leaders such as CEO, product managers, marketing managers, suppliers, distributors, and consumers are frequently interviewed. These interviews provide valuable insights which help us to have better market understanding besides validating our estimates and forecast.
Data Triangulation
Industry Analysis
|
Qualitative Data |
Quantitative Data (2017-2025) |
|
|