Preclinical CRO Market by Service and End User - Global Industry Analysis and Forecast to 2027
Published On : January 2018 Pages : 100 Category: Pharmaceuticals Report Code : HC01445
Industry Trend Analysis
Rising focus of life science companies on their core abilities combined with ascend in outsourcing of noncore capacities are additionally anticipated that would add to the development of preclinical CROs market. There has been a considerable ascent in R&D spending plan for beginning late -stage and early-stage drug improvement procedures, which is additionally anticipated that would add to the market growth. According to a report by the Servier Research Institute, 50.0% disappointments in preclinical stage are because of issues amid nonclinical toxicology testing. The expansion in disappointment because of toxicology tests is relied upon to impel the interest for preclinical CROs, in this manner adding to the worldwide preclinical CRO market.
Service Type Outlook and Trend Analysis
The service portion is classified into bioanalysis, toxicology testing, DMPK studies and other preclinical services. Toxicology testing held the biggest share in the worldwide preclinical CROs market and is foreseen to rule the fragment over the gauge time frame. Toxicology testing is a huge part of Investigational New Drug (IND)- empowering considers in the United States. The ascent in outsourcing of noncore preclinical investigations to CROs and the developing capacities of CROs to offer extra value add services are foreseen to drive the market over the gauge time frame.
End User Outlook and Trend Analysis
The end-use section of preclinical CRO market incorporates government, medicinal device companies, academic institutes and biopharmaceutical organizations. Biopharmaceutical organizations are the biggest end-use section of the preclinical CRO market. This fragment is anticipated to rule the preclinical CRO market amid the conjecture time frame. This is inferable from increment in pattern of outsourcing end-to-end services, particularly among the little and moderate size biopharmaceutical organizations that need adequate skill in preclinical period of medication advancement.
Regional Outlook and Trend Analysis
North America held the biggest share in the worldwide preclinical CRO market and is foreseen to command the market over the figure time frame. Existence of set up beginning time improvement CROs, for example, Charles River Laboratories and LabCorp; better nature of work; set up logical experience and intuition. The changing plan of action of MNC's outsourcing and increasing expense of R&D are foreseen to have brought about a preclinical outsourcing pattern in areas, for example, Asia Pacific, attributable to the cost-proficiency of CROs in nations, for example, China and India. Asia Pacific is foreseen to witness the quickest development in the worldwide preclinical CRO market.
Competitive Insights
The leading players in the market are PAREXEL, Charles River Laboratories, Envigo, LabCorp, Eurofins Scientific, PRA Health Sciences and Pharmaceutical Product Development. Life science companies are switching from a functional to strategic outsourcing model, and this shift is foreseen to raise the demand for big CROs providing end-to-end preclinical services.
The Preclinical CRO Market is segmented as follows-
By Type:
- Toxicology testing
- Bioanalysis and DMPK studies
- Other preclinical services
By End User:
- Biopharmaceutical companies
- Medical device companies
- Government and academic institutes
By Region
- North America
- U.S
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia-Pacific
- Japan
- China
- Australia
- India
- South Korea
- Rest of Asia Pacific
- Rest of the World
- Brazil
- South Africa
- Saudi Arabia
- United Arab Emirates
- Others
Some of the key questions answered by the report are:
- What was the market size in 2017 and forecast from 2017 to 2027?
- What will be the industry market growth from 2017 to 2027?
- What are the major drivers, restraints, opportunities, challenges, and industry trends and their impact on the market forecast?
- What are the major segments leading the market growth and why?
- Which are the leading players in the market and what are the major strategies adopted by them to sustain the market competition?
Market Classification
- Preclinical CRO Market, By Service, Estimates and Forecast, 2017-2027 ($Million)
- Bioanalysis and DMPK studies
- Toxicology testing
- Other preclinical services
- Preclinical CRO Market, By End Users, Estimates and Forecast, 2017-2027 ($Million)
- Biopharmaceutical companies
- Government and academic institutes
- Medical device companies
-
Preclinical CRO Market, By Region, Estimates and Forecast, 2017-2027 ($Million)
o North America
§North America Preclinical CRO Market, By Country
oU.S. Preclinical CRO Market
oCanada Preclinical CRO Market
oMexico Preclinical CRO Market
oEurope
§Europe Preclinical CRO Market, By Country
oGermany Preclinical CRO Market
oUK Preclinical CRO Market
oFrance Preclinical CRO Market
oRussia Preclinical CRO Market
oItaly Preclinical CRO Market
oRest of Europe Preclinical CRO Market
oAsia-Pacific
§Asia-Pacific Preclinical CRO Market, By Country
oChina Preclinical CRO Market
oJapan Preclinical CRO Market
oSouth Korea Preclinical CRO Market
oIndia Preclinical CRO Market
oSoutheast Asia Preclinical CRO Market
oRest of Asia-Pacific Preclinical CRO Market
oSouth America
§South America Preclinical CRO Market, By Country
oBrazil Preclinical CRO Market
oArgentina Preclinical CRO Market
oColumbia Preclinical CRO Market
oRest of South America Preclinical CRO Market
oMiddle East and Africa
§Middle East and Africa Preclinical CRO Market, By Country
oSaudi Arabia Preclinical CRO Market
oUAE Preclinical CRO Market
oEgypt Preclinical CRO Market
oNigeria Preclinical CRO Market
oSouth Africa Preclinical CRO Market
o Rest of MEA Preclinical CRO Market
Table of Contents
1. Introduction
1.1. Report Description
2. Executive Summary
2.1. Key Highlights
3. Market Overview
3.1. Introduction
3.1.1. Market Definition
3.1.2. Market Segmentation
3.2. Market Dynamics
3.2.1. Drivers
3.2.2. Restraints
3.2.3. Opportunities
4. Market Analysis by Regions
4.1. North America (United States, Canada and Mexico)
4.1.1. United States Market Status and Outlook (2017-2027)
4.1.2. Canada Market Status and Outlook (2017-2027)
4.1.3. Mexico Market Status and Outlook (2017-2027)
4.2. Europe (Germany, France, UK, Russia, Italy and Rest of Europe)
4.2.1. Germany Market Status and Outlook (2017-2027)
4.2.2. France Market Status and Outlook (2017-2027)
4.2.3. UK Market Status and Outlook (2017-2027)
4.2.4. Russia Market Status and Outlook (2017-2027)
4.2.5. Italy Market Status and Outlook (2017-2027)
4.2.6. Rest of Europe Market Status and Outlook (2017-2027)
4.3. Asia-Pacific (China, Japan, Korea, India, Southeast Asia and Rest of Asia-Pacific)
4.3.1. China Market Status and Outlook (2017-2027)
4.3.2. Japan Market Status and Outlook (2017-2027)
4.3.3. Korea Market Status and Outlook (2017-2027)
4.3.4. India Market Status and Outlook (2017-2027)
4.3.5. Southeast Asia Market Status and Outlook (2017-2027)
4.3.6. Rest of Asia-Pacific Market Status and Outlook (2017-2027)
4.4. South America (Brazil, Argentina, Columbia and Rest of South America)
4.4.1. Brazil Market Status and Outlook (2017-2027)
4.4.2. Argentina Market Status and Outlook (2017-2027)
4.4.3. Columbia Market Status and Outlook (2017-2027)
4.4.4. Rest of South America Market Status and Outlook (2017-2027)
4.5. Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria, South Africa and Rest of MEA)
4.5.1. Saudi Arabia Market Status and Outlook (2017-2027)
4.5.2. United Arab Emirates Market Status and Outlook (2017-2027)
4.5.3. Egypt Market Status and Outlook (2017-2027)
4.5.4. Nigeria Market Status and Outlook (2017-2027)
4.5.5. South Africa Market Status and Outlook (2017-2027)
4.5.6. Turkey Market Status and Outlook (2017-2027)
4.5.7. Rest of Middle East and Africa Market Status and Outlook (2017-2027)
5. Preclinical CRO Market, By Service
5.1. Introduction
5.2. Global Preclinical CRO Revenue and Market Share by Service (2017-2027)
5.2.1. Global Preclinical CRO Revenue and Revenue Share by Service (2017-2027)
5.3. Bioanalysis & DMPK studies
5.3.1. Global Bioanalysis & DMPK studies Revenue and Growth Rate (2017-2027)
5.4. Toxicology testing
5.4.1. Global Toxicology testing Revenue and Growth Rate (2017-2027)
5.5. Other preclinical services
5.5.1. Global Other preclinical services Revenue and Growth Rate (2017-2027)
6. Preclinical CRO Market, By End User
6.1. Introduction
6.2. Global Preclinical CRO Revenue and Market Share by End User (2017-2027)
6.2.1. Global Preclinical CRO Revenue and Revenue Share by End User (2017-2027)
6.3. Biopharmaceutical companies
6.3.1. Global Biopharmaceutical companies Revenue and Growth Rate (2017-2027)
6.4. Government and academic institutes
6.4.1. Global Government and academic institutes Revenue and Growth Rate (2017-2027)
6.5. Medical device companies
6.5.1. Global Medical device companies Revenue and Growth Rate (2017-2027)
7. Preclinical CRO Market, By Region
7.1. Introduction
7.2. Global Preclinical CRO Revenue and Market Share by Regions
7.2.1. Global Preclinical CRO Revenue by Regions (2017-2027)
7.3. North America Preclinical CRO by Countries
7.3.1. North America Preclinical CRO Revenue and Growth Rate (2017-2027)
7.3.2. North America Preclinical CRO Revenue (Million USD) by Countries (2017-2027)
7.3.3. United States
7.3.3.1. United States Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.3.4. Canada
7.3.4.1. Canada Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.3.5. Mexico
7.3.5.1. Mexico Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.4. Europe Preclinical CRO by Countries
7.4.1. Europe Preclinical CRO Revenue and Growth Rate (2017-2027)
7.4.2. Europe Preclinical CRO Revenue (Million USD) by Countries (2017-2027)
7.4.3. Germany
7.4.3.1. Germany Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.4.4. France
7.4.4.1. France Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.4.5. UK
7.4.5.1. UK Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.4.6. Russia
7.4.6.1. Russia Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.4.7. Italy
7.4.7.1. Italy Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.4.8. Rest of Europe
7.4.8.1. Rest of Europe Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.5. Asia-Pacific Preclinical CRO by Countries
7.5.1. Asia-Pacific Preclinical CRO Revenue and Growth Rate (2017-2027)
7.5.2. Asia-Pacific Preclinical CRO Revenue (Million USD) by Countries (2017-2027)
7.5.3. China
7.5.3.1. China Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.5.4. Japan
7.5.4.1. Japan Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.5.5. Korea
7.5.5.1. Korea Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.5.6. India
7.5.6.1. India Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.5.7. Southeast Asia
7.5.7.1. Southeast Asia Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.5.8. Rest of Asia-Pacific
7.5.8.1. Rest of Asia-Pacific Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.6. South America Preclinical CRO by Countries
7.6.1. South America Preclinical CRO Revenue and Growth Rate (2017-2027)
7.6.2. South America Preclinical CRO Revenue (Million USD) by Countries (2017-2027)
7.6.3. Brazil
7.6.3.1. Brazil Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.6.4. Argentina
7.6.4.1. Argentina Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.6.5. Columbia
7.6.5.1. Columbia Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.6.6. Rest of South America
7.6.6.1. Rest of South America Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.7. Middle East and Africa Preclinical CRO by Countries
7.7.1. Middle East and Africa Preclinical CRO Revenue and Growth Rate (2017-2027)
7.7.2. Middle East and Africa Preclinical CRO Revenue (Million USD) by Countries (2017-2027)
7.7.3. Saudi Arabia
7.7.3.1. Saudi Arabia Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.7.4. United Arab Emirates
7.7.4.1. United Arab Emirates Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.7.5. Egypt
7.7.5.1. Egypt Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.7.6. Nigeria
7.7.6.1. Nigeria Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.7.7. South Africa
7.7.7.1. South Africa Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.7.8. Turkey
7.7.8.1. Turkey Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
7.7.9. Rest of Middle East and Africa
7.7.9.1. Rest of Middle East and Africa Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
8. Company Profiles
8.1. Charles River Laboratories
8.1.1. Business Overview
8.1.2. Service Portfolio
8.1.3. Strategic Developments
8.1.4. Financial Overview
8.2. Envigo Corporation
8.2.1. Business Overview
8.2.2. Service Portfolio
8.2.3. Strategic Developments
8.2.4. Financial Overview
8.3. Eurofins Scientific
8.3.1. Business Overview
8.3.2. Service Portfolio
8.3.3. Strategic Developments
8.3.4. Financial Overview
8.4. PRA Health Sciences, Inc
8.4.1. Business Overview
8.4.2. Service Portfolio
8.4.3. Strategic Developments
8.4.4. Financial Overview
8.5. Wuxi AppTec
8.5.1. Business Overview
8.5.2. Service Portfolio
8.5.3. Strategic Developments
8.5.4. Financial Overview
8.6. Medpace, Inc.
8.6.1. Business Overview
8.6.2. Service Portfolio
8.6.3. Strategic Developments
8.6.4. Financial Overview
8.7. Pharmaceutical Product Development (PPD), LLC
8.7.1. Business Overview
8.7.2. Service Portfolio
8.7.3. Strategic Developments
8.7.4. Financial Overview
8.8. PARAXEL International Corporation
8.8.1. Business Overview
8.8.2. Service Portfolio
8.8.3. Strategic Developments
8.8.4. Financial Overview
8.9. ICON Plc.
8.9.1. Business Overview
8.9.2. Service Portfolio
8.9.3. Strategic Developments
8.9.4. Financial Overview
8.10. Laboratory Corporation of America, Inc.
8.10.1. Business Overview
8.10.2. Service Portfolio
8.10.3. Strategic Developments
8.10.4. Financial Overview
9. Preclinical CRO Market Forecast (2017-2027)
9.1. Global Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2027)
9.2. Preclinical CRO Market Forecast by Regions (2017-2027)
9.2.1. North America Preclinical CRO Market Forecast (2017-2027)
9.2.1.1. United States Preclinical CRO Market Forecast (2017-2027)
9.2.1.2. Canada Preclinical CRO Market Forecast (2017-2027)
9.2.1.3. Mexico Preclinical CRO Market Forecast (2017-2027)
9.2.2. Europe Preclinical CRO Market Forecast (2017-2027)
9.2.2.1. Germany Preclinical CRO Market Forecast (2017-2027)
9.2.2.2. France Preclinical CRO Market Forecast (2017-2027)
9.2.2.3. UK Preclinical CRO Market Forecast (2017-2027)
9.2.2.4. Russia Preclinical CRO Market Forecast (2017-2027)
9.2.2.5. Italy Preclinical CRO Market Forecast (2017-2027)
9.2.2.6. Rest of Europe Preclinical CRO Market Forecast (2017-2027)
9.2.3. Asia-Pacific Preclinical CRO Market Forecast (2017-2027)
9.2.3.1. China Preclinical CRO Market Forecast (2017-2027)
9.2.3.2. Japan Preclinical CRO Market Forecast (2017-2027)
9.2.3.3. Korea Preclinical CRO Market Forecast (2017-2027)
9.2.3.4. India Preclinical CRO Market Forecast (2017-2027)
9.2.3.5. Southeast Asia Preclinical CRO Market Forecast (2017-2027)
9.2.3.6. Rest of Asia-Pacific Preclinical CRO Market Forecast (2017-2027)
9.2.4. South America Preclinical CRO Market Forecast (2017-2027)
9.2.4.1. Brazil Preclinical CRO Market Forecast (2017-2027)
9.2.4.2. Argentina Preclinical CRO Market Forecast (2017-2027)
9.2.4.3. Columbia Preclinical CRO Market Forecast (2017-2027)
9.2.4.4. Rest of South America Preclinical CRO Market Forecast (2017-2027)
9.2.5. Middle East and Africa Preclinical CRO Market Forecast (2017-2027)
9.2.5.1. Saudi Arabia Preclinical CRO Market Forecast (2017-2027)
9.2.5.2. United Arab Emirates Preclinical CRO Market Forecast (2017-2027)
9.2.5.3. Egypt Preclinical CRO Market Forecast (2017-2027)
9.2.5.4. Nigeria Preclinical CRO Market Forecast (2017-2027)
9.2.5.5. South Africa Preclinical CRO Market Forecast (2017-2027)
9.2.5.6. Turkey Preclinical CRO Market Forecast (2017-2027)
9.2.5.7. Rest of Middle East and Africa Preclinical CRO Market Forecast (2017-2027)
9.3. Preclinical CRO Market Forecast by Service (2017-2027)
9.3.1. Preclinical CRO Forecast by Service (2017-2027)
9.3.2. Preclinical CRO Market Share Forecast by Service (2017-2027)
9.4. Preclinical CRO Market Forecast by End User (2017-2027)
9.4.1. Preclinical CRO Forecast by End User (2017-2027)
9.4.2. Preclinical CRO Market Share Forecast by End User (2017-2027)
List of Tables
Figure United States Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Canada Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Mexico Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Germany Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure France Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure UK Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Russia Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Italy Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Rest of Europe Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure China Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Japan Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Korea Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure India Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Southeast Asia Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Rest of Asia-Pacific Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Brazil Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Argentina Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Columbia Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Rest of South America Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Saudi Arabia Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure United Arab Emirates Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Egypt Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Nigeria Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure South Africa Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Turkey Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Figure Rest of Middle East and Africa Preclinical CRO Revenue (Million USD) and Growth Rate (2017-2025)
Table Global Preclinical CRO Revenue and Revenue Share by Service (2017-2018)
Figure Global Bioanalysis & DMPK studies Revenue and Growth Rate (2017-2018)
Figure Global Toxicology testing Revenue and Growth Rate (2017-2018)
Figure Global Other preclinical services Revenue and Growth Rate (2017-2018)
Table Global Preclinical CRO Revenue and Revenue Share by End User (2017-2018)
Figure Global Biopharmaceutical companies Revenue and Growth Rate (2017-2018)
Figure Global Government and academic institutes Revenue and Growth Rate (2017-2018)
Figure Global Medical device companies Revenue and Growth Rate (2017-2018)
Table Global Preclinical CRO Revenue by Regions (2017-2018)
Figure North America Preclinical CRO Growth Rate (2017-2018)
Figure North America Preclinical CRO Revenue and Growth Rate (2017-2018)
Figure North America Preclinical CRO by Countries (2017-2018)
Figure North America Preclinical CRO Revenue (Million USD) by Countries (2017-2018)
Figure United States Preclinical CRO Growth Rate (2017-2018)
Figure United States Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Canada Preclinical CRO Growth Rate (2017-2018)
Figure Canada Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Mexico Preclinical CRO Growth Rate (2017-2018)
Figure Mexico Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Europe Preclinical CRO Growth Rate (2017-2018)
Figure Europe Preclinical CRO Revenue and Growth Rate (2017-2018)
Figure Europe Preclinical CRO by Countries (2017-2018)
Figure Europe Preclinical CRO Revenue (Million USD) by Countries (2017-2018)
Figure Germany Preclinical CRO Growth Rate (2017-2018)
Figure Germany Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure France Preclinical CRO Growth Rate (2017-2018)
Figure France Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure UK Preclinical CRO Growth Rate (2017-2018)
Figure UK Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Russia Preclinical CRO Growth Rate (2017-2018)
Figure Russia Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Italy Preclinical CRO Growth Rate (2017-2018)
Figure Italy Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Rest of Europe Preclinical CRO Growth Rate (2017-2018)
Figure Rest of Europe Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Asia-Pacific Preclinical CRO Growth Rate (2017-2018)
Figure Asia-Pacific Preclinical CRO Revenue and Growth Rate (2017-2018)
Figure Asia-Pacific Preclinical CRO by Countries (2017-2018)
Figure Asia-Pacific Preclinical CRO Revenue (Million USD) by Countries (2017-2018)
Figure China Preclinical CRO Growth Rate (2017-2018)
Figure China Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Japan Preclinical CRO Growth Rate (2017-2018)
Figure Japan Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Korea Preclinical CRO Growth Rate (2017-2018)
Figure Korea Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure India Preclinical CRO Growth Rate (2017-2018)
Figure India Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Southeast Asia Preclinical CRO Growth Rate (2017-2018)
Figure Southeast Asia Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Rest of Asia-Pacific Preclinical CRO Growth Rate (2017-2018)
Figure Rest of Asia-Pacific Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure South America Preclinical CRO Growth Rate (2017-2018)
Figure South America Preclinical CRO Revenue and Growth Rate (2017-2018)
Figure South America Preclinical CRO by Countries (2017-2018)
Figure South America Preclinical CRO Revenue (Million USD) by Countries (2017-2018)
Figure Brazil Preclinical CRO Growth Rate (2017-2018)
Figure Brazil Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Argentina Preclinical CRO Growth Rate (2017-2018)
Figure Argentina Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Columbia Preclinical CRO Growth Rate (2017-2018)
Figure Columbia Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Rest of South America Preclinical CRO Growth Rate (2017-2018)
Figure Rest of South America Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Middle East and Africa Preclinical CRO Growth Rate (2017-2018)
Figure Middle East and Africa Preclinical CRO Revenue and Growth Rate (2017-2018)
Figure Middle East and Africa Preclinical CRO by Countries (2017-2018)
Figure Middle East and Africa Preclinical CRO Revenue (Million USD) by Countries (2017-2018)
Figure Saudi Arabia Preclinical CRO Growth Rate (2017-2018)
Figure Saudi Arabia Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure United Arab Emirates Preclinical CRO Growth Rate (2017-2018)
Figure United Arab Emirates Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Egypt Preclinical CRO Growth Rate (2017-2018)
Figure Egypt Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Nigeria Preclinical CRO Growth Rate (2017-2018)
Figure Nigeria Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure South Africa Preclinical CRO Growth Rate (2017-2018)
Figure South Africa Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Turkey Preclinical CRO Growth Rate (2017-2018)
Figure Turkey Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Figure Rest of Middle East and Africa Preclinical CRO Growth Rate (2017-2018)
Figure Rest of Middle East and Africa Preclinical CRO Revenue (Millions USD) and Growth Rate (2017-2018)
Table Charles River Laboratories Preclinical CRO Financial Overview
Table Envigo Corporation Preclinical CRO Financial Overview
Table Eurofins Scientific Preclinical CRO Financial Overview
Table PRA Health Sciences, Inc Preclinical CRO Financial Overview
Table Wuxi AppTec Preclinical CRO Financial Overview
Table Medpace, Inc. Preclinical CRO Financial Overview
Table Pharmaceutical Product Development (PPD), LLC Preclinical CRO Financial Overview
Table PARAXEL International Corporation Preclinical CRO Financial Overview
Table ICON Plc. Preclinical CRO Financial Overview
Table Laboratory Corporation of America, Inc. Preclinical CRO Financial Overview
Figure Global Preclinical CRO Revenue (Millions USD) and Growth Rate (2018-2025)
Table Preclinical CRO Market Forecast by Regions (2018-2025)
Figure North America Preclinical CRO Market Forecast (2018-2025)
Figure United States Preclinical CRO Market Forecast (2018-2025)
Figure Canada Preclinical CRO Market Forecast (2018-2025)
Figure Mexico Preclinical CRO Market Forecast (2018-2025)
Figure Europe Preclinical CRO Market Forecast (2018-2025)
Figure Germany Preclinical CRO Market Forecast (2018-2025)
Figure France Preclinical CRO Market Forecast (2018-2025)
Figure UK Preclinical CRO Market Forecast (2018-2025)
Figure Russia Preclinical CRO Market Forecast (2018-2025)
Figure Italy Preclinical CRO Market Forecast (2018-2025)
Figure Rest of Europe Preclinical CRO Market Forecast (2018-2025)
Figure Asia-Pacific Preclinical CRO Market Forecast (2018-2025)
Figure China Preclinical CRO Market Forecast (2018-2025)
Figure Japan Preclinical CRO Market Forecast (2018-2025)
Figure Korea Preclinical CRO Market Forecast (2018-2025)
Figure India Preclinical CRO Market Forecast (2018-2025)
Figure Southeast Asia Preclinical CRO Market Forecast (2018-2025)
Figure Rest of Asia-Pacific Preclinical CRO Market Forecast (2018-2025)
Figure South America Preclinical CRO Market Forecast (2018-2025)
Figure Brazil Preclinical CRO Market Forecast (2018-2025)
Figure Argentina Preclinical CRO Market Forecast (2018-2025)
Figure Columbia Preclinical CRO Market Forecast (2018-2025)
Figure Rest of South America Preclinical CRO Market Forecast (2018-2025)
Figure Middle East and Africa Preclinical CRO Market Forecast (2018-2025)
Figure Saudi Arabia Preclinical CRO Market Forecast (2018-2025)
Figure United Arab Emirates Preclinical CRO Market Forecast (2018-2025)
Figure Egypt Preclinical CRO Market Forecast (2018-2025)
Figure Nigeria Preclinical CRO Market Forecast (2018-2025)
Figure South Africa Preclinical CRO Market Forecast (2018-2025)
Figure Turkey Preclinical CRO Market Forecast (2018-2025)
Figure Rest of Middle East and Africa Preclinical CRO Market Forecast (2018-2025)
Figure Global Preclinical CRO Forecast by Service (2018-2025)
Figure Global Preclinical CRO Market Share Forecast by Service (2018-2025)
Figure Global Preclinical CRO Forecast by Service (2018-2025)
Figure Global Preclinical CRO Forecast by End User (2018-2025)
Figure Global Preclinical CRO Market Share Forecast by End User (2018-2025)
Figure Global Preclinical CRO Forecast by End User (2018-2025)
Please Note: Data related to the Companies are subject to Availability.
Research Methodology
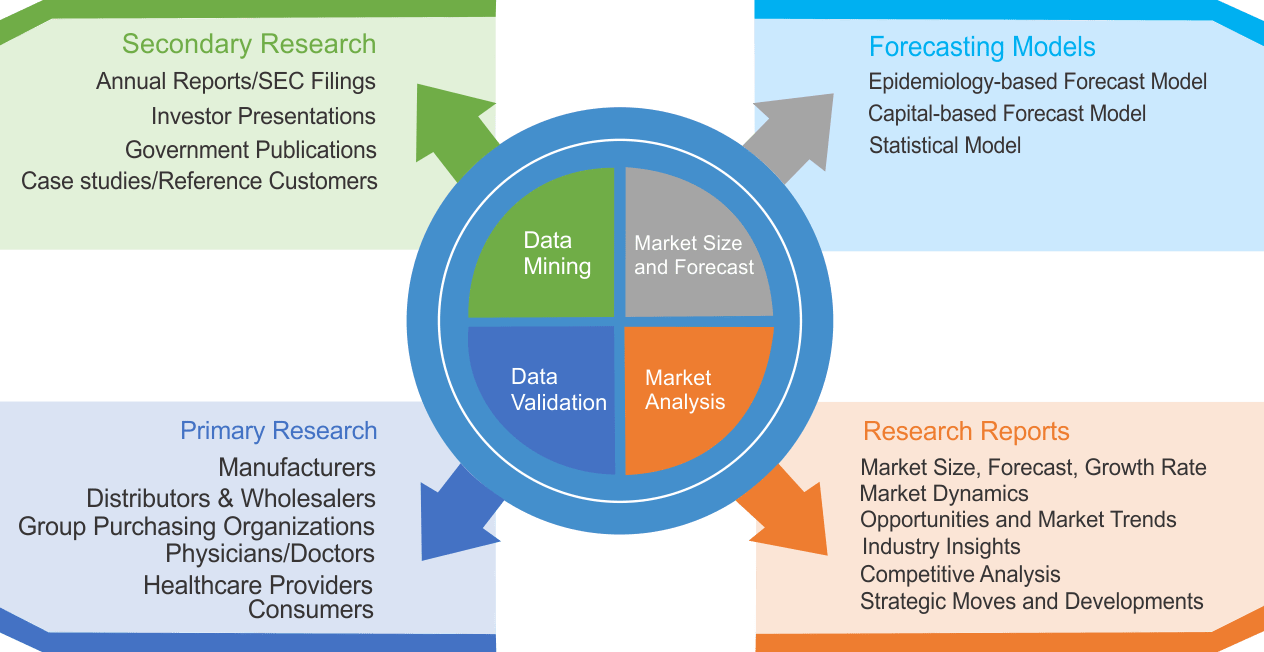
We use both primary as well as secondary research for our market surveys, estimates and for developing forecast. Our research process commence by analyzing the problem which enable us to design the scope for our research study. Our research process is uniquely designed with enough flexibility to adjust according to changing nature of products and markets, while retaining core element to ensure reliability and accuracy in research findings. We understand both macro and micro-economic factors to evaluate and forecast different market segments.
Data Mining
Data is extensively collected through various secondary sources such as annual reports, investor presentations, SEC filings, and other corporate publications. We also refer trade magazines, technical journals, paid databases such as Factiva and Bloomberg, industry trade journals, scientific journals, and social media data to understand market dynamics and industry trends. Further, we also conduct primary research to understand market drivers, restraints, opportunities, challenges, and competitive scenario to build our analysis.
Data Collection Matrix
|
Data Collection Matrix |
Supply Side |
Demand Side |
|
Primary Data Sources |
|
|
|
Secondary Data Sources |
|
|
Market Modeling and Forecasting
We use epidemiology and capital equipment-based models to forecast market size of different segments at country and regional level.
- Epidemiology-based Forecasting Model: This method uses epidemiology data gathered through various publications and from physicians to estimate population of patients, flow of treatment of individual disease and therapies. The data collected through this method includes statics on incidence of disease, population suffering from disease, and treatment population. This method is used to understand:
- Number of patients for particular device or medical procedure and
- Repeated use of particular device depending on health and condition of patient
- Capital-based Forecasting Model: This method of forecasting is based on number of replacements, installed-based and new sales of capital equipment used in various healthcare and diagnostic centers. These three parameters are calculated and forecast is developed. Installation base is calculated as average number of units per facility; while sales for particular year is calculated from number of new and replace units. Secondary data is collected through various supply chain intermediaries and opinion leaders to arrive at installation and sales rate. These techniques help our analysts in validating market and developed market estimates and forecast.
We do forecast on basis of several parameters such as market drivers, market opportunities, industry trends government regulations, raw materials supply and trade dynamics to ensure relevance of forecast with market scenario. With increasing need to granulized information, we used bottom-up methodology for forecasting where we evaluate each regional segment differently and combined all forecast to develop final market forecast.
Data Validation
We believe primary research is a very important tool in analyzing and forecasting different markets. In order to make sure accuracy of our findings, our team conducts primary interviews at every stage of research to gain deep insights into current business environment and future trends and key developments in market. This includes use of various methods such as telephonic interviews, focus groups, face to face interviews and questionnaires to validate our research from all aspects. We validate our data through primary research from key industry leaders such as CEO, product managers, marketing managers, suppliers, distributors, and consumers are frequently interviewed. These interviews provide valuable insights which help us to have better market understanding besides validating our estimates and forecast.
Data Triangulation
Industry Analysis
|
Qualitative Data |
Quantitative Data (2017-2025) |
|
|