Small-bore Connectors Market By Type (liquid Bore Connectors And Gas Bore Connectors) And End User (hospitals, Clinics And Ambulatory Surgical Centers)- Global Industry Analysis And Forecast To 2023
Published On : July 2018 Pages : 190 Category: Medical Devices Report Code : HC071102
Industry Outlook and Trend Analysis
The Small Bore Connectors Market is expected to witness considerable growth during the forecast period. Small bore connectors give a system to the association between an assortment of therapeutic gadgets incorporating those with enteral and non-enteral (e.g., intravenous) applications. Small bore connectors characterized as having an interior measurement of less than 8.5 mm, and AAMI/ANSI/ISO 80369-1 standard portrays the Luer connector as a little bore "conelike fitting with a 6% decrease for syringes, needles, and certain other therapeutic gear." Luer connectors have a male and a female part that are joined to frame a safe, yet separable watertight association. The association accomplished by utilization of a push fitting (a Luer slip) or fasten strung fitting (a Luer bolt) that joins the male and the female decreased fittings. AAMI/ANSI/ISO 80369-1 gives general prerequisites to small-bore connectors exhaust connectors for fluids and gasses in social insurance applications.
Drivers & Restrains
To make their small bore connectors more secure, makers are creating gadgets with new connectors that adjust to worldwide gauges. As makers change to new items, they are changing between time progresses connectors to encourage the coherence of care. While the FDA urges makers to fuse the new connector outline gauges for enteral gadgets and other future gadget composes, the agency does not expect makers to change to the new standard plan or to expel items right now being used from the market. Devices with more seasoned outlines (legacy devices) may at present be accessible available for patients who depend on them for their care. Every one of these variables goes about as drivers in the development of the small bore connectors market.
Regional Outlook and Trend Analysis
Europe is overwhelming in the worldwide small Bore Connectors predominantly because of the nearness of solid players in this locale. Europe took after by North America and APAC districts, they both are developing at the high pace because of expanding government bolster for producers. Development in the Central East and African district is extensively less yet at the same time with critical development.
Competitive Insights
The leading players in the market are Hopf A. Kunststoff-verarbeitung GmbH, Sigma-Aldrich Co. LLC, CPC and others. The major players in the market are profiled in detail in view of qualities, for example, company portfolio, business strategies, financial overview, recent developments, and share of the overall industry.
The Small Bore Connectors Market is segmented as follows-
By Type:
- Liquid Bore Connectors
- Gas Bore Connectors
By End User:
- Hospitals
- Clinics
- Ambulatory Surgical Centres
By Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Russia
- Italy
- Rest of Europe
- Asia-Pacific
- China
- Japan
- South Korea
- India
- Southeast Asia
- Rest of Asia-Pacific
- South America
- Brazil
- Argentina
- Columbia
- South Africa
- Rest of South America
- Middle East and Africa
- Saudi Arabia
- UAE
- Egypt
- Nigeria
- South Africa
- Rest of MEA
Some of the key questions answered by the report are:
- What was the market size in 2017 and forecast from 2017 to 2023?
- What will be the industry market growth from 2017 to 2023?
- What are the major drivers, restraints, opportunities, challenges, and industry trends and their impact on the market forecast?
- What are the major segments leading the market growth and why?
- Which are the leading players in the market and what are the major strategies adopted by them to sustain the market competition?
Market Classification
· Small-Bore Connectors Market, By Type, Estimates and Forecast, 2014-2023 ($Million)
· Liquid Bore Connectors
· Gas Bore Connectors
· Small-Bore Connectors Market, By End User, Estimates and Forecast, 2014-2023 ($Million)
· Hospitals
· Clinics
· Ambulatory Surgical Centers
· Small-Bore Connectors Market, By Region, Estimates and Forecast, 2014-2023 ($Million)
· North America
§ North America Small-Bore Connectors Market, By Country
o U.S. Small-Bore Connectors Market
o Canada Small-Bore Connectors Market
o Mexico Small-Bore Connectors Market
· Europe
§ Europe Small-Bore Connectors Market, By Country
o Germany Small-Bore Connectors Market
o UK Small-Bore Connectors Market
o France Small-Bore Connectors Market
o Russia Small-Bore Connectors Market
o Italy Small-Bore Connectors Market
o Rest of Europe Small-Bore Connectors Market
· Asia-Pacific
§ Asia-Pacific Small-Bore Connectors Market, By Country
o China Small-Bore Connectors Market
o Japan Small-Bore Connectors Market
o South Korea Small-Bore Connectors Market
o India Small-Bore Connectors Market
o Southeast Asia Small-Bore Connectors Market
o Rest of Asia-Pacific Small-Bore Connectors Market
· South America
§ South America Small-Bore Connectors Market
o Brazil Small-Bore Connectors Market
o Argentina Small-Bore Connectors Market
o Columbia Small-Bore Connectors Market
o South Africa Small-Bore Connectors Market
o Rest of South America Small-Bore Connectors Market
· Middle East and Africa
§ Middle East and Africa Small-Bore Connectors Market
o Saudi Arabia Small-Bore Connectors Market
o UAE Small-Bore Connectors Market
o Egypt Small-Bore Connectors Market
o Nigeria Small-Bore Connectors Market
o South Africa Small-Bore Connectors Market
o Rest of MEA Small-Bore Connectors Market
Table of Contents
1. Introduction
1.1. Report Description
1.2. Research Methodology
1.2.1. Secondary Research
1.2.2. Primary Research
2. Executive Summary
2.1. Key Highlights
3. Market Overview
3.1. Introduction
3.1.1. Market Definition
3.1.2. Market Segmentation
3.2. Market Dynamics
3.2.1. Drivers
3.2.2. Restraints
3.2.3. Opportunities
3.2.3.1. Emerging Markets to Offer Lucrative Growth Opportunities
4. Market Analysis by Regions
4.1. North America (United States, Canada and Mexico)
4.1.1. United States Market States and Outlook (2017-2023)
4.1.2. Canada Market States and Outlook (2017-2023)
4.1.3. Mexico Market States and Outlook (2017-2023)
4.2. Europe (Germany, France, UK, Russia, Italy and Rest of Europe)
4.2.1. Germany Market States and Outlook (2017-2023)
4.2.2. France Market States and Outlook (2017-2023)
4.2.3. UK Market States and Outlook (2017-2023)
4.2.4. Russia Market States and Outlook (2017-2023)
4.2.5. Italy Market States and Outlook (2017-2023)
4.2.6. Rest of Europe Market States and Outlook (2017-2023)
4.3. Asia-Pacific (China, Japan, Korea, India, Southeast Asia and Rest of Asia-Pacific)
4.3.1. China Market States and Outlook (2017-2023)
4.3.2. Japan Market States and Outlook (2017-2023)
4.3.3. Korea Market States and Outlook (2017-2023)
4.3.4. India Market States and Outlook (2017-2023)
4.3.5. Rest of Asia-Pacific Market States and Outlook (2017-2023)
4.4. South America (Brazil, Argentina, Columbia and Rest of South America)
4.4.1. Brazil Market States and Outlook (2017-2023)
4.4.2. Argentina Market States and Outlook (2017-2023)
4.4.3. Columbia Market States and Outlook (2017-2023)
4.4.4. Rest of South America Market States and Outlook (2017-2023)
4.5. Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria, South Africa and Rest of MEA)
4.5.1. Saudi Arabia Market States and Outlook (2017-2023)
4.5.2. UAE Market States and Outlook (2017-2023)
4.5.3. Egypt Market States and Outlook (2017-2023)
4.5.4. Nigeria Market States and Outlook (2017-2023)
4.5.5. South Africa Market States and Outlook (2017-2023)
4.5.6. Rest of MEA Market States and Outlook (2017-2023)
5. Small-Bore Connectors Market, By Type
5.1. Introduction
5.2. Global Small-Bore Connectors Sales, Revenue and Market Share by Type (2017-2027)
5.2.1. Global Small-Bore Connectors Sales and Sales Share by Type (2017-2027)
5.2.2. Global Small-Bore Connectors Revenue and Revenue Share by Type (2017-2027)
5.3. Liquid Bore Connectors
5.3.1. Global Liquid Bore Connectors Sales and Growth Rate (2017-2027)
5.4. Gas Bore Connectors
5.4.1. Global Gas Bore Connectors Sales and Growth Rate (2017-2027)
6. Small-Bore Connectors Market, By End User
6.1. Introduction
6.2. Global Small-Bore Connectors Sales, Revenue and Market Share by End User (2017-2027)
6.2.1. Global Small-Bore Connectors Sales and Sales Share by End User (2017-2027)
6.2.2. Global Small-Bore Connectors Revenue and Revenue Share by End User (2017-2027)
6.3. Hospitals
6.3.1. Global Hospitals Sales and Growth Rate (2017-2027)
6.4. Clinics
6.4.1. Global Clinics Sales and Growth Rate (2017-2027)
6.5. Ambulatory Surgical Centers
6.5.1. Global Ambulatory Surgical Centers Sales and Growth Rate (2017-2027)
7. Small-Bore Connectors Market, By Region
7.1. Introduction
7.2. Global Small-Bore Connectors Sales, Revenue and Market Share by Regions
7.2.1. Global Small-Bore Connectors Sales by Regions (2017-2027)
7.2.2. Global Small-Bore Connectors Revenue by Regions (2017-2027)
7.3. North America Small-Bore Connectors by Countries
7.3.1. North America Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.3.2. North America Small-Bore Connectors Revenue and Growth Rate (2017-2027)
7.3.3. North America Small-Bore Connectors Sales by Countries (2017-2027)
7.3.4. North America Small-Bore Connectors Revenue (Million USD) by Countries (2017-2027)
7.3.5. U.S.
7.3.5.1. United States Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.3.5.2. United States Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.3.6. Canada
7.3.6.1. Canada Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.3.6.2. Canada Small-Bore Connectors Sales (Millions USD) and Growth Rate (2017-2027)
7.3.7. Mexico
7.3.7.1. Mexico Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.3.7.2. Mexico Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.4. Europe Small-Bore Connectors by Countries
7.4.1. Europe Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.4.2. Europe Small-Bore Connectors Revenue and Growth Rate (2017-2027)
7.4.3. Europe Small-Bore Connectors Sales by Countries (2017-2027)
7.4.4. Europe Small-Bore Connectors Revenue (Million USD) by Countries (2017-2027)
7.4.5. Germany
7.4.5.1. Germany Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.4.5.2. Germany Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.4.6. UK
7.4.6.1. UK Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.4.6.2. UK Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.4.7. France
7.4.7.1. France Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.4.7.2. France Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.4.8. Russia
7.4.8.1. Russia Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.4.8.2. Russia Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.4.9. Italy
7.4.9.1. Italy Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.4.9.2. Italy Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.4.10. Rest of Europe
7.4.10.1. Rest of Europe Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.4.10.2. Rest of Europe Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.5. Asia-Pacific
7.5.1. Asia-Pacific Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.5.2. Asia-Pacific Small-Bore Connectors Revenue and Growth Rate (2017-2027)
7.5.3. Asia-Pacific Small-Bore Connectors Sales by Countries (2017-2027)
7.5.4. Asia-Pacific Small-Bore Connectors Revenue (Million USD) by Countries (2017-2027)
7.5.5. China
7.5.5.1. China Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.5.5.2. China Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.5.6. Japan
7.5.6.1. Japan Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.5.6.2. Japan Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.5.7. Korea
7.5.7.1. Korea Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.5.7.2. Korea Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.5.8. India
7.5.8.1. India Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.5.8.2. India Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.5.9. Southeast Asia
7.5.9.1. Southeast Asia Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.5.9.2. Southeast Asia Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.5.10. Rest of Asia-Pacific
7.5.10.1. Rest of Asia-Pacific Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.5.10.2. Rest of Asia-Pacific Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.6. South America
7.6.1. South America Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.6.2. South America Small-Bore Connectors Revenue and Growth Rate (2017-2027)
7.6.3. South America Small-Bore Connectors Sales by Countries (2017-2027)
7.6.4. South America Small-Bore Connectors Revenue (Million USD) by Countries (2017-2027)
7.6.5. Brazil
7.6.5.1. Brazil Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.6.5.2. Brazil Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.6.6. Argentina
7.6.6.1. Argentina Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.6.6.2. Argentina Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.6.7. Columbia
7.6.7.1. Columbia Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.6.7.2. Columbia Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.6.8. Rest of South America
7.6.8.1. Rest of South America Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.6.8.2. Rest of South America Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.7. Middle East and Africa
7.7.1. Middle East and Africa Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.7.2. Middle East and Africa Small-Bore Connectors Revenue and Growth Rate (2017-2027)
7.7.3. Middle East and Africa Small-Bore Connectors Sales by Countries (2017-2027)
7.7.4. Middle East and Africa Small-Bore Connectors Revenue (Million USD) by Countries (2017-2027)
7.7.5. Saudi Arabia
7.7.5.1. Saudi Arabia Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.7.5.2. Saudi Arabia Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.7.6. United Arab Emirates
7.7.6.1. United Arab Emirates Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.7.6.2. United Arab Emirates Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.7.7. Egypt
7.7.7.1. Egypt Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.7.7.2. Egypt Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.7.8. Nigeria
7.7.8.1. Nigeria Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.7.8.2. Nigeria Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.7.9. South Africa
7.7.9.1. South Africa Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.7.9.2. South Africa Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
7.7.10. Rest of Middle East and Africa
7.7.10.1. Rest of Middle East and Africa Small-Bore Connectors Sales and Growth Rate (2017-2027)
7.7.10.2. Rest of Middle East and Africa Small-Bore Connectors Revenue (Millions USD) and Growth Rate (2017-2027)
8. Company Profiles
8.1. Hopf A. Kunststoff-verarbeitung GmbH
8.1.1. Business Overview
8.1.2. Product Portfolio
8.1.3. Strategic Developments
8.1.4. Sales, Revenue and Market Share
8.2. CPC
8.2.1. Business Overview
8.2.2. Product Portfolio
8.2.3. Strategic Developments
8.2.4. Sales, Revenue and Market Share
8.3. Elcam Medical
8.3.1. Business Overview
8.3.2. Product Portfolio
8.3.3. Strategic Developments
8.3.4. Sales, Revenue and Market Share
8.4. Sigma-Aldrich Co. LLC
8.4.1. Business Overview
8.4.2. Product Portfolio
8.4.3. Strategic Developments
8.4.4. Sales, Revenue and Market Share
9. Global Small-Bore Connectors Market Competition, by Manufacturer
9.1. Global Small-Bore Connectors Sales and Market Share by Manufacturer (2017-2017)
9.2. Global Small-Bore Connectors Revenue and Market Share by Manufacturer (2017-2017)
9.3. Global Small-Bore Connectors Price by Manufacturer (2017-2017)
9.4. Top 5 Small-Bore Connectors Manufacturer Market Share
9.5. Market Competition Trend
10. Small-Bore Connectors Market Forecast (2027-2023)
10.1. Global Small-Bore Connectors Sales, Revenue (Millions USD) and Growth Rate (2027-2023)
10.2. Small-Bore Connectors Market Forecast by Regions (2027-2023)
10.2.1. North America Small-Bore Connectors Market Forecast (2027-2023)
10.2.1.1. United States Small-Bore Connectors Market Forecast (2027-2023)
10.2.1.2. Canada Small-Bore Connectors Market Forecast (2027-2023)
10.2.1.3. Mexico Small-Bore Connectors Market Forecast (2027-2023)
10.2.2. Europe Small-Bore Connectors Market Forecast (2027-2023)
10.2.2.1. Germany Small-Bore Connectors Market Forecast (2027-2023)
10.2.2.2. United Kingdom Small-Bore Connectors Market Forecast (2027-2023)
10.2.2.3. France Small-Bore Connectors Market Forecast (2027-2023)
10.2.2.4. Russia Small-Bore Connectors Market Forecast (2027-2023)
10.2.2.5. Italy Small-Bore Connectors Market Forecast (2027-2023)
10.2.2.6. Rest of the Europe Small-Bore Connectors Market Forecast (2027-2023)
10.2.3. Asia-Pacific Small-Bore Connectors Market Forecast (2027-2023)
10.2.3.1. China Small-Bore Connectors Market Forecast (2027-2023)
10.2.3.2. Japan Small-Bore Connectors Market Forecast (2027-2023)
10.2.3.3. Korea Small-Bore Connectors Market Forecast (2027-2023)
10.2.3.4. India Small-Bore Connectors Market Forecast (2027-2023)
10.2.3.5. Southeast Asia Small-Bore Connectors Market Forecast (2027-2023)
10.2.3.6. Rest of Asia-Pacific Small-Bore Connectors Market Forecast (2027-2023)
10.2.4. South America Small-Bore Connectors Market Forecast (2027-2023)
10.2.4.1. Brazil Small-Bore Connectors Market Forecast (2027-2023)
10.2.4.2. Argentina Small-Bore Connectors Market Forecast (2027-2023)
10.2.4.3. Columbia Small-Bore Connectors Market Forecast (2027-2023)
10.2.4.4. Rest of South America Small-Bore Connectors Market Forecast (2027-2023)
10.2.5. Middle East and Africa Small-Bore Connectors Market Forecast (2027-2023)
10.2.5.1. Saudi Arabia Small-Bore Connectors Market Forecast (2027-2023)
10.2.5.2. UAE Small-Bore Connectors Market Forecast (2027-2023)
10.2.5.3. Egypt Small-Bore Connectors Market Forecast (2027-2023)
10.2.5.4. Nigeria Small-Bore Connectors Market Forecast (2027-2023)
10.2.5.5. South Africa Small-Bore Connectors Market Forecast (2027-2023)
10.2.5.6. Rest of MEA Small-Bore Connectors Market Forecast (2027-2023)
10.3. Small-Bore Connectors Market Forecast by Type (2027-2023)
10.3.1. Global Small-Bore Connectors Sales Forecast by Type (2027-2023)
10.3.2. Global Small-Bore Connectors Sales Market Share Forecast by Type (2027-2023)
10.4. Small-Bore Connectors Market Forecast by End User (2027-2023)
10.4.1. Global Small-Bore Connectors Sales Forecast by End User (2027-2023)
10.4.2. Global Small-Bore Connectors Sales Market Share Forecast by End User (2027-2023)
List of Tables
*You can glance through the list of Tables and Figures when you view the sample copy of Small-Bore Connectors Market.
Research Methodology
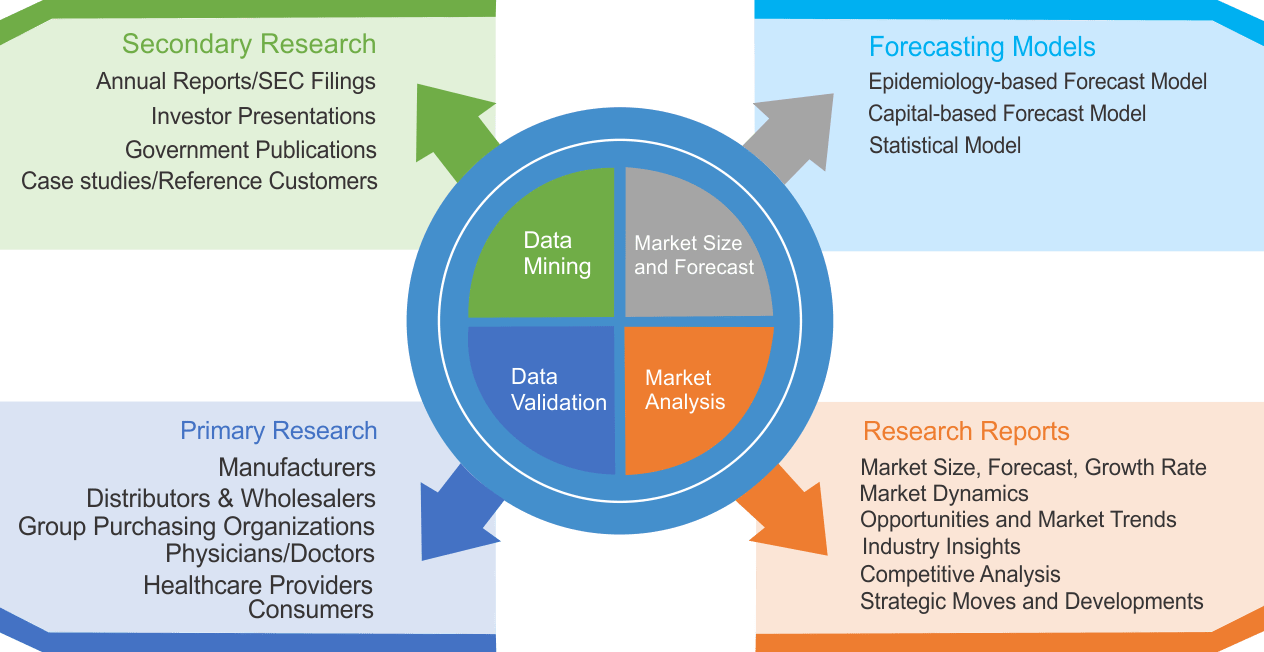
We use both primary as well as secondary research for our market surveys, estimates and for developing forecast. Our research process commence by analyzing the problem which enable us to design the scope for our research study. Our research process is uniquely designed with enough flexibility to adjust according to changing nature of products and markets, while retaining core element to ensure reliability and accuracy in research findings. We understand both macro and micro-economic factors to evaluate and forecast different market segments.
Data Mining
Data is extensively collected through various secondary sources such as annual reports, investor presentations, SEC filings, and other corporate publications. We also refer trade magazines, technical journals, paid databases such as Factiva and Bloomberg, industry trade journals, scientific journals, and social media data to understand market dynamics and industry trends. Further, we also conduct primary research to understand market drivers, restraints, opportunities, challenges, and competitive scenario to build our analysis.
Data Collection Matrix
|
Data Collection Matrix |
Supply Side |
Demand Side |
|
Primary Data Sources |
|
|
|
Secondary Data Sources |
|
|
Market Modeling and Forecasting
We use epidemiology and capital equipment-based models to forecast market size of different segments at country and regional level.
- Epidemiology-based Forecasting Model: This method uses epidemiology data gathered through various publications and from physicians to estimate population of patients, flow of treatment of individual disease and therapies. The data collected through this method includes statics on incidence of disease, population suffering from disease, and treatment population. This method is used to understand:
- Number of patients for particular device or medical procedure and
- Repeated use of particular device depending on health and condition of patient
- Capital-based Forecasting Model: This method of forecasting is based on number of replacements, installed-based and new sales of capital equipment used in various healthcare and diagnostic centers. These three parameters are calculated and forecast is developed. Installation base is calculated as average number of units per facility; while sales for particular year is calculated from number of new and replace units. Secondary data is collected through various supply chain intermediaries and opinion leaders to arrive at installation and sales rate. These techniques help our analysts in validating market and developed market estimates and forecast.
We do forecast on basis of several parameters such as market drivers, market opportunities, industry trends government regulations, raw materials supply and trade dynamics to ensure relevance of forecast with market scenario. With increasing need to granulized information, we used bottom-up methodology for forecasting where we evaluate each regional segment differently and combined all forecast to develop final market forecast.
Data Validation
We believe primary research is a very important tool in analyzing and forecasting different markets. In order to make sure accuracy of our findings, our team conducts primary interviews at every stage of research to gain deep insights into current business environment and future trends and key developments in market. This includes use of various methods such as telephonic interviews, focus groups, face to face interviews and questionnaires to validate our research from all aspects. We validate our data through primary research from key industry leaders such as CEO, product managers, marketing managers, suppliers, distributors, and consumers are frequently interviewed. These interviews provide valuable insights which help us to have better market understanding besides validating our estimates and forecast.
Data Triangulation
Industry Analysis
|
Qualitative Data |
Quantitative Data (2017-2025) |
|
|